Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Effects of Cannabinolic Acid Amides on TNF-Α and IL-6 Expression in Lipopolysaccharide Activated Human Peripheral Blood Mononuclear Cells
*Corresponding author:Alexander Aizikovich, AL&AM Pharmachem Ltd. Carmel st. 5/35, Rehovot, 76305, Israel.
Received:May 18, 2022; Published: May 24, 2022
DOI: 10.34297/AJBSR.2022.16.002221
Abstract
Introduction: The effects of tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) amide derivatives 1-9 on
TNF-𝛼 and IL-6 expression and cell proliferation were investigated on lipopolysaccharide (LPS) activated human peripheral blood
mononuclear cells (PBMCs).
Materials and Methods: Compounds 1-9 were tested on LPS activated human PBMCs and several inflammatory endpoints
monitored. Negative controls included PBMC only, LPS and dexamethasone with LPS. After a 72-hour incubation, PBMC supernatants
were collected to measure IL-6 and TNF-α levels by ELISA. Cell pellets were harvested, and cell proliferation analyzed with the CTG
assay.
Results: Compounds 1 and 2 increased TNF-α expression and inhibited cell proliferation, while unsubstituted amides 5, 6
decreased the production of TNF-α compared to LPS-treated cells. None of the compounds tested had a noticeable effect on LPS
induced IL-6 expression.
Conclusion: The change of substitution in the amide group yields THCA and CBDA derivatives with different and sometimes
opposing activities with respect to TNF-𝛼 expression with a general lack of effect on IL-6 output. The function of several of these compounds as potential activators of the immune response to viral infections and antitumor activity, as well as their
immunosuppressive potential warrants further investigation and may highlight the contributions of such novel cannabinolic acidbased
drugs to pharmacology.
Keywords: Cannabinoids, THCA, TNF-𝛼, IL-6, LPS, PBMCs
Introduction
The spread of the Covid-19 virus in the world has significantly intensified research into effective treatments for this disease. Since progression of the disease includes two stages - infection with the virus and activation of host immunity, with further dissemination of the infection, a “cytokine storm” arises which is characterized by an uncontrolled release of inflammatory cytokines, predominantly involving TNF-α. and IL-6. Unfortunately, the main drugs used against COVID-19 do not provide effective treatment. The search for new drugs that can effectively and quickly block the expression of TNFα, IL-6 and other inflammatory cytokines has therefore become the focus of the fight against this severe disease [1].
The use of cannabinoids as new potential anti-inflammatory drugs for the treatment of COVID-19 has drawn the attention of researchers from the beginning of the pandemic [2-4]. Cannabinoids bind to cannabinoid receptors CB1 and CB2 thereby promoting the expression of pro- and anti-inflammatory cytokines in immune cells. To date cannabinoid studies have predominantly focused on tetrahydrocannabinol, and particularly on cannabidiol, as these are the most affordable and stable products isolated from marijuana [5]. The potential medical uses of their precursors tetrahydrocannabinolic (THCA) and cannabidiolic (CBDA) acids have been disregarded because their pure forms are difficult to isolate, and they tend to decarboxylate. It is only recently that several studies have been devoted to their biological activities their ability to inhibit the release of cyclooxygenases during inflammation [6,7].
The great opportunities, that may be captured by the synthetic transformation of the carboxyl group, also presage the significance of these acids as potential novel drug precursors [8,9]. This is particularly relevant in the context of the new technology which allows to extract these acids from plant materials using ionexchange resins and yields a purity suitable for further synthesis [10]. The current work is an activity study of several of these previously described THCA and CBDA amides, specifically focusing on their effects on LPS-induced TNF-𝛼 and IL-6 secretion as well as on proliferation of human PBMCs. The choice of TNF-𝛼 and IL-6 was based on widespread reports of their significant contribution to cytokine pro-inflammatory activities during the “cytokine storm” induced by COVID-19 viral infection [11].
Materials and Methods
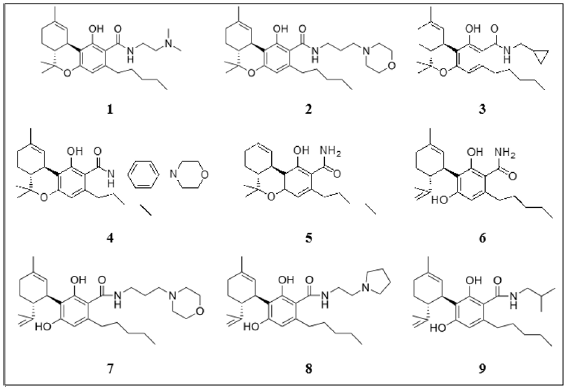
Compounds 1-9 were obtained from THCA and CBDA [10] in accordance with methods of their synthesis [8].
(1’R,2’R)-2,6-dihydroxy-5’-methyl-4-pentyl-2’-(prop- 1-en-2-yl)-1’,2’,3’,4’-tetrahydro-[1,1’-biphenyl]-3- carboxamide 6
Yield 48%. 1H NMR (CDCl3), δ: 0.88-0.91(3H, t, CH); 1.33- 1.35(4H, m, CH); 1.62-1.65(3H, m, CH); 1.70(3H, s, CH); 1.78- 1.99(5H, m, CH); 2.10-2.17(2H, bd, CH); 2.2.19-2.23(1H, m, CH); 2.36-2.45(1H, m, CH); 2.71-2.7.4(2H, m, CH); 4.08(1H, bs, CH); 4.39(1H, s, CH); 4.52(1H, s, CH); 5.58(1H, s, CH); 5.94(2H, s, NH); 6.41(1H, s, CH); 11.94(1H, s, OH). 13C NMR (CDCl3), d: 13.98, 18.87, 22.45, 23.71, 27.86, 30.25, 30.85, 31.78, 35.21, 46.63, 107.57, 110.39, 111.15, 115.096, 124.08, 140.13, 140.83, 147.36, 158.83, 161.17, 175.31. Molecular ions [M-H] +=358 were observed, consistent with the molecular formula C22H31NO3. Effects of these compounds were tested on human PBMCs challenged with Lipopolysaccharide (LPS) by monitoring several inflammatory endpoints.
Materials and Equipment
VICTOR Nivo Multimode Microplate Reader (PerkinElmer, VICTOR Nivo); Lymphoprep™ (Stemcell, Cat. No. SV30010, Lot. No. J170012); SepMateTM-50 (STEMCELL Technologies, Cat. No. A10491-01); Lipopolysaccharides from Escherichia coli O111:B4 (Sigma, Cat. No L2630-10MG); Dexamethasone (Alfa Aesar, Cat. No A17590); Human TNF-a ELISA (Biolegend, Cat. No 430204); Corning® 96-well Flat Clear Bottom White (Corning, Cat. No 3903); Cell Titer-Glo® Luminescent Cell Viability Assay (Promega, Cat. No G7572). Blood samples were collected from healthy volunteers who had signed the Informed Consent Form (ICF) according to protocols reviewed and approved by Chempartner Institutional Ethic Committee (IEC).
PBMC Isolation
Human blood samples from individual donors were diluted with an equal volume of sterile Phosphate Buffered Saline (PBS) and mixed by gentle agitation. Fifteen milliliters of Lymphoprep medium were transferred into a new 50 mL centrifuge tube, whilst ensuring that the Ficoll and blood (30 mL) volume ratio was kept at 1:2 and the diluted blood sample was then carefully added onto the surface of the Ficoll medium. Centrifugation resulted in the appearance of four layers. From top to bottom of the tube these layers corresponded to plasma, mononuclear cells, Ficoll medium and RBCs. After removing the plasma, mononuclear cells were transferred into a new sterile centrifuge tube. Sterile PBS buffer was added to the collected PBMCs for washing at a volume ratio of 3:1. PBMCs were washed with 5-10 mL PBS twice more before counting cells with a cytometer.
Cells were centrifuged at 350x g, 10 min, 20°C, with an acceleration and deceleration setting of 5 and resuspended in RPMI 1640 complete medium (containing 10% Fetal Bovine Serum FBS) for the assay. Cell density was adjusted to a final concentration of 2E6 cells/mL with RPMI 1640 complete medium. Fresh PBMC cell suspensions (100 μL/well) were seeded into 96-well plates (#3903) and a dilution series of compounds and 0.1 μg/mL LPS added to each well (in triplicates). Controls included 3 wells with PBMCs only, 3 wells with 0.1 μg/mL LPS and 2 wells with 1 μg/ mL dexamethasone (Dex) and 0.1 μg/mL LPS. After 72 hours of incubation, the supernatant was collected for Tumor Necrosis Factor-α (TNF-α) measurement by Enzyme-linked Immunosorbent Assay (ELISA), and cell pellets were harvested to measure cell proliferation using the CellTiter-Glo® Luminescent Cell Viability Assay (CTG).
Preparation of Compound Solutions
Compounds were diluted with RPMI 1640 complete medium according to the layout. Compounds were diluted to a working concentration of 10 μM with RPMI 1640 complete medium and further diluted in two 10-fold serial dilution steps. Compounds were prepared at a 5-fold concentration (50 μM). Diluted compounds (50 μL/well) were added to the indicated wells, and plates incubated at 37 °C, 5% CO2 incubator for 72 hrs.
LPS induced cytokine release assay: LPS (0.1 μg/mL, 100 μL) was added to each well. Dex (1 μg/mL) was included as a positive control. Plates were incubated at 37 °C, 5% CO2 for 72 hrs. Supernatant was collected to measure IL-6 and TNF-α by ELISA. Cell proliferation was measured by CTG.
Statistical Analysis
Data analysis was performed using the Graphpad Prism 6.0 software. Data is represented as mean value with standard error of the mean (SEM). Data of all experiments presented in (Figures 1-3) are one-way ANOVA analyses compared to the LPS treated group.
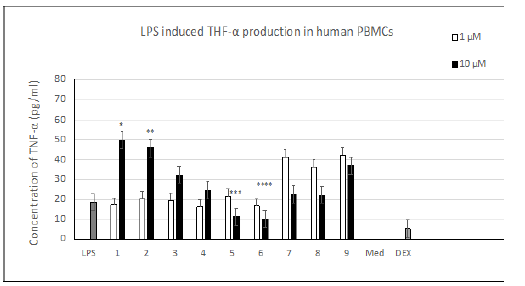
Figure 1:Influence of the amino group structures of compounds 1-9 on LPS induced TNF-α production (pg/ml) in human PBMCs (LPS – 0.1 μg/ml; DEX – 1.0 μg/ml + 0.1 μg/ml LPS; One-way ANOVA analysis: *p, **p, ***p, ****p <0.01 when compared to the LPS treated group; n=3.
Results
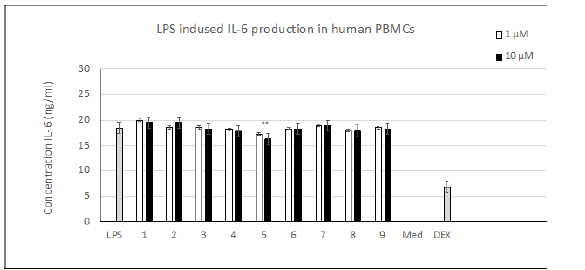
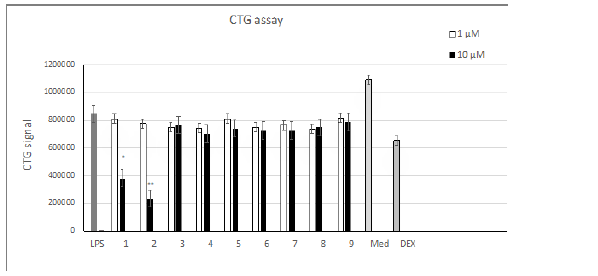
Compounds 1-9 were obtained by amination of the carbonyl group of the THCA and CBDA aromatic rings [8] (Table 1). Evaluation of TNF-α levels induced by LPS. As shown on Figure 1, the addition of 0.1 μg/mL LPS induced an obvious increase in the TNF-α level in human PBMCs. THCA derivatives 1-4 promoted the production of TNF-α compared to LPS-treated cells. Compound 5 and its CBDA analog, compound 6, resulted in a dose-dependent decrease in TNF-α. Compounds 7-9 increased the production of TNF-α at a concentration of 1 μm and decreased TNF-α levels at a concentration of 10 μM. Evaluating IL-6 levels induced by LPS. As shown on Figure 2 the addition of 0.1 μg/mL of LPS induced an obvious increase in the level of IL-6 in human PBMCs, as was the case for TNF-α. Compound 5 dose-dependently decreased the production of IL-6 compared to LPS-treated cells, but this value did not exceed 10% at a compound concentration of 10 μM. The remaining substances did not have any noticeable effects. Cell proliferation in the LPS induced PBMC system. As shown in (Figure 3), at 10 μM, compounds 1 and 2 inhibited cell proliferation 2.5 to 3-fold compared to LPS-control cells. The remaining compounds show a slight decrease in toxicity compared to dexamethasone.
Discussion
TNF-𝛼 is a pro-inflammatory cytokine and appears d uring an injury response or bacterial and viral infections. Other important features are its ability to cause hemorrhagic necrosis of tumors in vivo in combination with its ability to kill tumor cells in vitro [12]. IL-6 is another major proinflammatory cytokine with a variety of effects on the immune system, particularly in the development of long-term immune activation and contributes to the progression of the COVID-19 induced “cytokine storm” [11]. IL-6 also plays an important role in the onset and development of many types of cancers such as pancreatic and colorectal cancers [13].
The structure of the amide substituent in compounds 1-9 had a significant effect on the expression of TNF-𝛼 (Figure 1). According to the data obtained, all compounds examined can be subdivided into two groups depending on the change in TNF-𝛼 expression induced by compound concentrations of 1μM and 10 μM. The first group includes THCA derivatives 1-4, the second – unsubstituted THCA amide 5 and CBDA amides 6-9.
The ability of compounds 1 and 2 to induce the expression of TNF-𝛼 together with their inhibition of cell proliferation (Figure 3) is consistent with the previously reported antitumor activity of these compounds [8,14]. The role of TNF-𝛼 in tumor suppression was discussed right from its discovery in 1975, but to date its function as an anticancer agent remains problematic [15,16]. TNF-𝛼 is also released by CBDA amides 7-9 at a concentration of 1 μM with further suppression at 10 μM. These dual results obtained for compounds 7-9 suggest that these compounds may act via several mechanisms, as was the case for CBD and its analogues with a 2,2-dimethylheptyl substituent [17,18]. Compounds 5, 6 exhibited quite a pronounced suppression of TNF-𝛼 expression, which together with their low cytotoxicity may mark these compounds as potentially useful antiinflammatory drugs. The data presented in (Figure 2), shows that except for compound 5 none of the cannabinoids tested altered the expression of proinflammatory cytokine IL-6. A slight decrease (of up to 10%) depending on the concentration tested was observed in the case of unsubstituted THCA amide 5.
Conclusion
Changing the substitution of the amide group of THCA and CBDA derivatives resulted in different and sometimes opposing activities with respect to the expression of TNF-𝛼, with a general lack of effect on IL-6 output. The function of several of these compounds as potential activators of the immune response to viral infections and antitumor activity, as well as their immunosuppressive potential warrants further investigation and may highlight the contributions of such novel cannabinolic acid-based drugs to pharmacology.
Acknowledgment
The author thanks the employees of the Shanghai Chempartner (China) for their high level of professionalism and attention to his work.
Author Disclosure Statements
The author has no conflicts of interests, and no competing financial interests exist.
Funding Information
This work was funded by the AL&AM Pharmachem Ltd. company’s own funds (Grant ALAM 002-2020).
References
- Beth Russell, Charlotte Moss, Gincy George, Aida Santaolalla, Andrew Cope, et al. (2020) Associations between immune-suppressive and stimulating drugs and novel COVID-19 - a systematic review of current evidence. Ecancer 14: 1022.
- Myriam El Biali, Barbara Broers, Marie Besson, Jules Demeules (2020) Cannabinoids and COVID-19. Med Cannabis Cannabinoids 3(2): 111–115.
- Nicole Paland, Antonina Pechkovsky, Miran Aswad, Haya Hamza, Tania Popov, et al. (2021) The Immunopathology of COVID-19 and the Cannabis Paradigm. Frontiers in Immunology 12: 631233.
- Natascia Bruni, Carlo Della Pepa, Simonetta Oliaro Bosso, Enrica Pessione, Daniela Gastaldi, et al. (2018) Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecule. 23(10): 2478.
- Jean-Gilles L, Gran B, Constantinescu CS (2010) Interaction between cytokines, cannabinoids and the nervous system. Immunobiology 215: 606–610.
- Guillermo Moreno-Sanz (2016) Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of D9-Tetrahydrocannabinolic Acid A. Cannabis and Cannabinoid Research 1(1): 124-130.
- Runaak LR, Felth J, Karlsson PC, et al. (2011) Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis sativa. Biol. Pharm. Bull 34(5): 774-778.
- Alexander Aizikovich. Cannabinolic acid derivatives and uses thereof. Patent US 20210087159.
- Alexander Aizikovich (2021) In vitro activity of novel cannabinoids derived from tetrahydrocannabinolic acid on various human tumor cell lines. Journal of Oncology Research 3:55-59.
- Alexander Aizikovich. Process for purification of tetrahydrocannabinolic and cannabidiolic acids from plant material extract. Patent US 2021292295.
- Anna Kovalchuk, Bo Wang, Dongping Li, Rocio Rodriguez-Juarez, Slava Ilnytskyy, et al. (2021) Fighting the storm: could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19? AGING 13(2): 1571-1590.
- P Ghezzi, Cerami (2005) Tumor necrosis factor as a pharmacological target. Molecular Biotechnology 31(3): 239-44.
- Bellone G, Smirne C, Angelo F, et al. (2006) Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother 55(6): 684-698.
- Aizikovich A (2020) Anticancer Effect of New Cannabinoids Derived from Tetrahydrocannabinolic Acid on PANC-1 and AsPC-1 Human Pancreas Tumor Cells. J Pancreatic Cancer 6(1): 40-44.
- Anne Montfort, Céline Colacios, Thierry Levade, Nathalie Andrieu-Abadie, Nicolas Meyer, et al. (2019) The TNF paradox in cancer progression and immunotherapy. Front Immun 10: 1818.
- Steven F Josephs, Thomas E Ichim, Stephen M Prince, Santosh Kesari, Francesco M Marincola, et al. (2018) Unleashing endogenous TNF‑alpha as a cancer immunotherapeutic. J Transl Med 16(1): 242.
- James M Nichols, Barbara LF Kaplan (2020) Immune Responses Regulated by Cannabidiol. Cannabis and Cannabinoid Research 5(1): 12-31.
- Ben Shabat S, Hanus LO Katzavian G (2006) New Cannabidiol Derivatives: Synthesis, Binding to Cannabinoid Receptor, and Evaluation of Their Anti-inflammatory Activity. J Med Chem 49(3): 1113-1117.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.